Though initially considered primarily a respiratory disease, as the pandemic has evolved, coronavirus disease 2019 (COVID-19) has been increasingly implicated in heart injury. Reports indicate cardiac damage in over 20% of patients, with evidence of direct viral injury, thromboembolism with ischemic complications (circulating clot that causes an obstruction in the blood vessels), and cytokine storm (excessive activation of the immune system). These patients are at significantly higher risk of dying from COVID-19, but it’s not clear how infection leads to these injuries.
The Bruce McManus Cardiovascular Biobank (BMCB) team (Paul Hanson et al), recently studied the explanted hearts of 21 COVID-19 positive decedents. Using a custom tissue microarray on regions of pathological interest and immunohistochemistry and in situ hybridization, they compared these hearts to clinically matched controls and patients with other causes of viral myocarditis.

The COVID-19 samples displayed signs of direct and indirect viral injury (see figure below), demonstrating the multifactorial nature of COVID-19 injury.
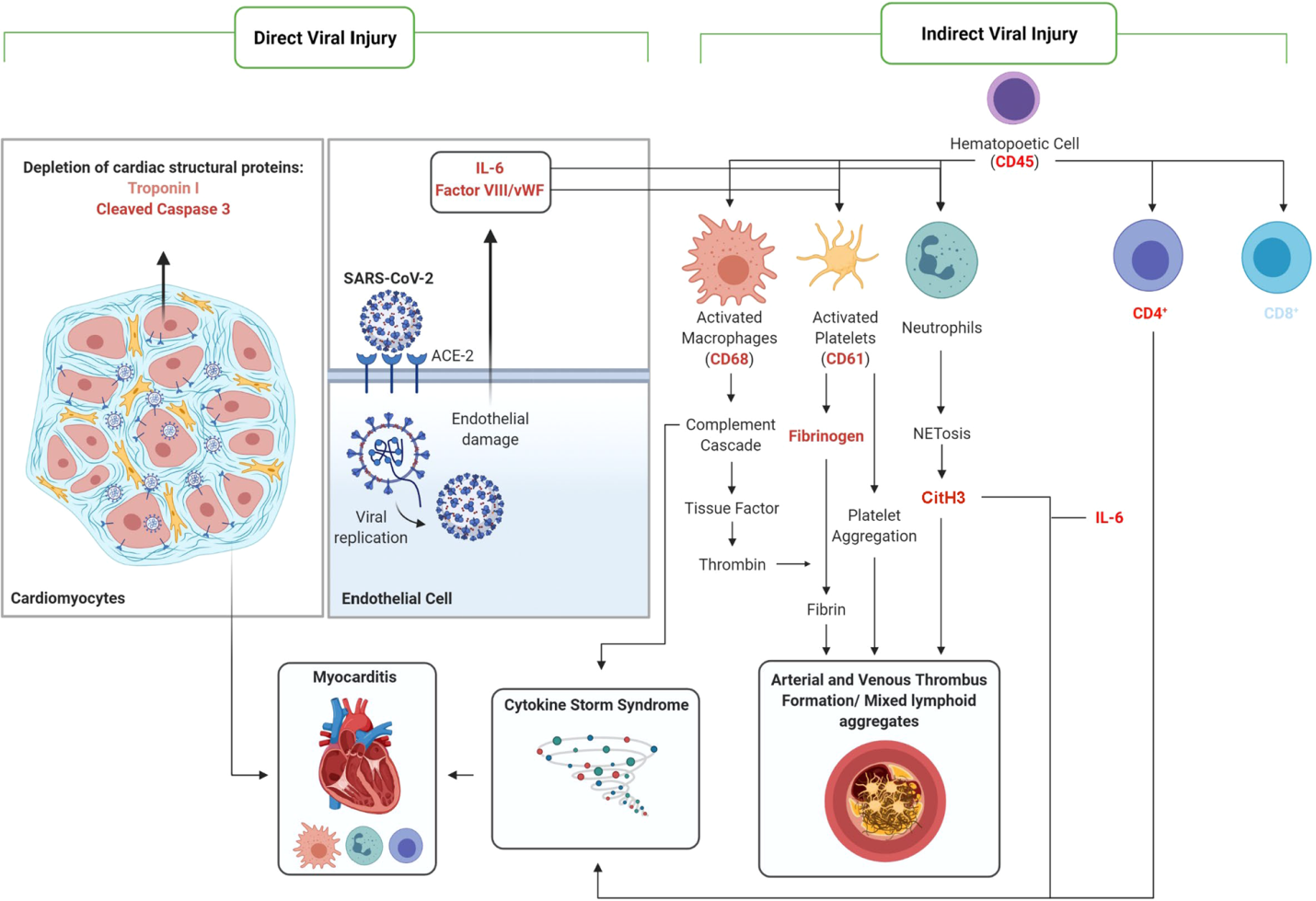
Signs of direct injury included depleted troponin and increased cleaved caspase-3; these markers may be helpful in prognosing and diagnosing COVID-19 heart failure in the future.
Indirect mechanisms of injury, including clots in the arteries and veins, inflammation of the blood vessels, and enhanced blood vessel formation, were unique to the COVID-19 samples, and not observed in other virus-associated heart failure samples.
Other observations included the presence of Neutrophil extracellular traps (NETs) in the heart tissue of all COVID-19 patients, regardless of injury degree, and borderline myocarditis (inflammation without associated injury to the muscle cells of the heart) in 19/21 patients.
This work was highlighted in a feature publication in Laboratory Investigation, with the cover showing the characteristic histopathologic features of COVID-19-associated cardiac injury in critically ill patients.